Leading Healthcare Investors
Supporting the Development of Oral Medications
Our Investors

Become One of Our Healthcare Investors
Partner with us in developing new oral medications for hypercholesterolemia and other metabolic diseases. Give us a call to schedule an appointment with our team. We hope to do business with you!
Our Events and Presentations
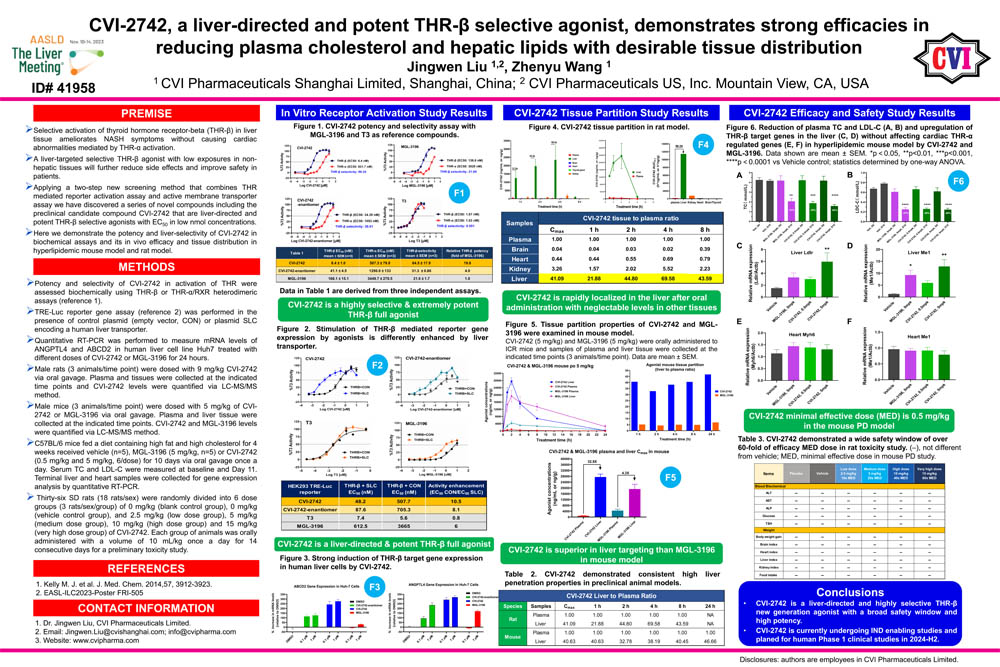
11/11/2023 - Poster Presentation (ID# 41958) at The Liver Meeting 2023 November 10-14, Boston, MA.
12/22/2021 - CVI Pharmaceuticals (Shanghai) Limited announced that the randomized, double-blind, multi-dose, placebo-controlled phase II clinical study to evaluate the efficacy and safety of CVI-LM001 tablet in hypercholesterolemia subjects has completed all subjects enrollment.
The main purpose of the study is to evaluate the reduction of LDL-C from baseline in different dose groups of CVI-LM001 compared with placebo after 12 weeks of treatment, and to evaluate the safety and tolerability of CVI-LM001 in different dose groups.10/26/2021 - CVI Pharmaceuticals (Shanghai) Limited and Yangtze River Pharmaceuticals Group have entered into a China exclusive licensing agreement for CVI-LM001, a first-in-class LDL-cholesterol lowering new drug and an oral PCSK9 synthesis inhibitor, being developed to treat patients with hypercholesterolemia.
Under terms of the agreement, CVI will receive upfront license fee, development, regulatory and sales milestone payments and certain annual nationwide net sales following marketing approval of CVI-LM001 in China. Yangtze River Pharmaceuticals Group is responsible for all development and regulatory activities and costs beyond those associated with the ongoing Phase 2 study.
CVI Pharmaceuticals keeps global extra-China exclusive rights to CVI-LM001 for therapies of hypercholesterolemia and other cardio-metabolic diseases.11/13/2020 - Poster Presentation at American Heart Association’s Scientific Sessions 2020 November 14-16, Dallas, TX.
11/13/2020 - Poster Presentation at The Liver Meeting 2020 November 13-17, Boston, MA.
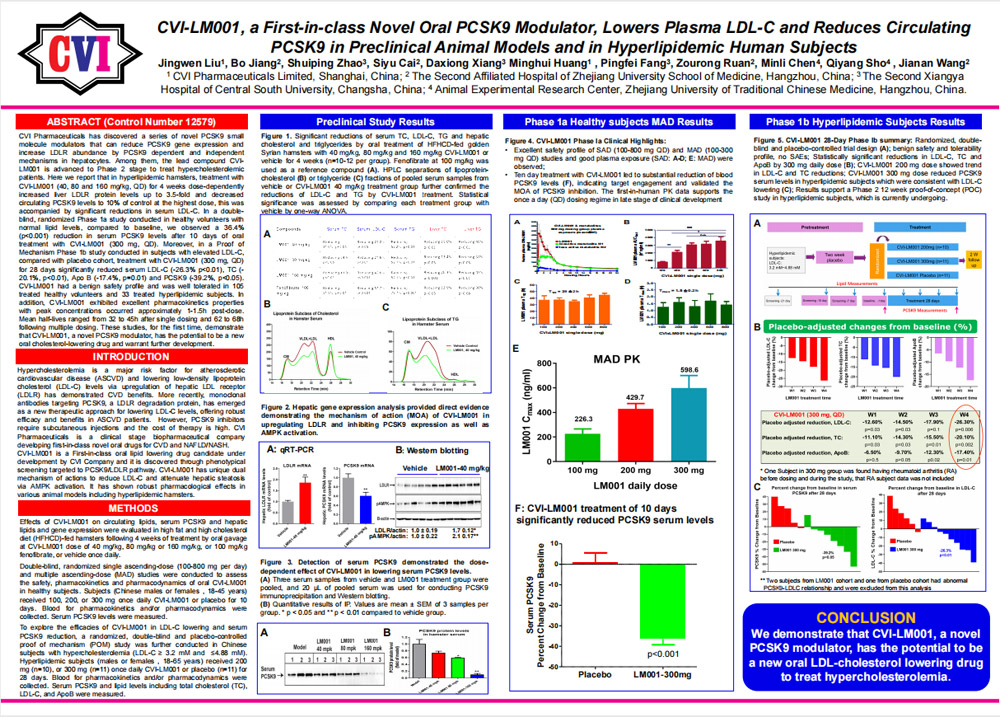
04/22/2020 - Abstract submitted to American Heart Association’s Scientific Sessions 2020 November 14-16, Dallas, TX. Abstract title: CVI-LM001, a First-in-Class Novel Oral PCSK9 Modulator, Lowers Plasma LDL-C and Reduces Circulating PCSK9 in Preclinical Animal Models and in Hyperlipidemic Human Subjects.
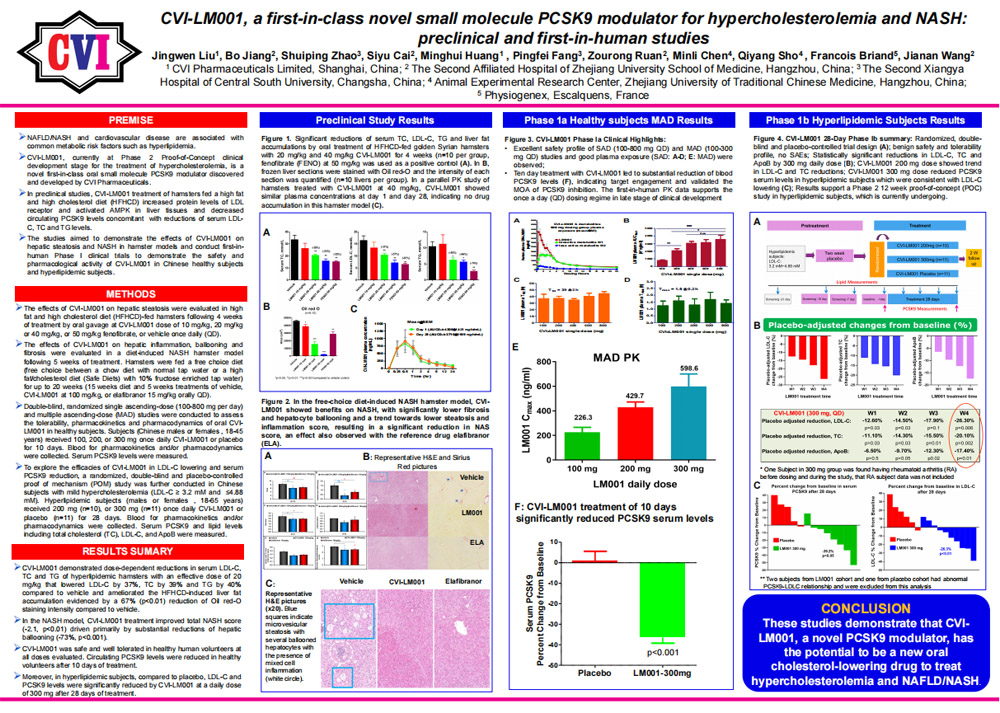
04/13/2020 - Abstract submitted to The Liver Meeting 2020 November 13-17, Boston, MA. Abstract title: CVI-LM001, a First-in-Class Novel Small Molecule PCSK9 Modulator for Hypercholesterolemia and NASH: Preclinical and First-in-Human Studies.
01/14/2020 - Oral Presentation at 37th Annual J.P. Morgan Biotech Conference, San Francisco, CA, USA.
CVI Pharmaceuticals Doses First Patient with Primary Hypercholesterolemia in a 12-Week Proof-of-Concept Trial with CVI-LM001, an oral, First-in-Class, small molecule PCSK9 Inhibitor
SAN DIEGO, August 19, 2020 –CVI Pharmaceuticals Inc., a clinical-stage biotechnology company focused on building an innovative pipeline of first-in-class drugs to treat hypercholesterolemia and NASH/NAFLD diseases, today announced that the first patient has been dosed in its 12-week, Phase 2 Proof-of-Concept (POC) trial of CVI-LM001, a sustained, PCSK9 inhibitor small molecule, in patients with primary hypercholesterolemia.This Phase 2 POC clinical trial is designed to evaluate the safety, tolerability and change in LDL-C with orally administered 100-300 mg (QD) of CVI-LM001. The study is a randomized, double-blind, parallel-dosing, multi-center, placebo-controlled trial led by Jianan Wang, MD, PhD, Chair of Department of Cardiology and the President of the second affiliated hospital of Zhejiang University School of Medicine, and the Principal Coordinating Investigator of the study. Key analyses include evaluating effects of treatment with CVI-LM001 on lipid panel including total cholesterol, LDL-C, HDL-C, ApoB, non-HDL, triglyceride, Lp(a), hsCRP and plasma PCSK9 level.
Standard-of-care, lipid-lowering statins are effective at lowering LDL-C, leading to well-documented CV benefits. However, not all patients can tolerate statins or reach their LDL-C goal on maximally-tolerated statin-dosing. Patients with atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolemia (HeFH) who require additional LDL-C lowering on top of maximally tolerated statin therapy represent a high-risk patient population with an unmet medical need. Currently, there are two approved injectable PCSK9 inhibitors (Repatha and Praluent) which can significantly reduce LDL-C levels and improve cardiovascular outcomes. However, injectable dose regimen, poor compliance and expensive drug price prevent patients with hypercholesterolemia access to these drugs. Thus, development of once daily, orally administered small molecule PCSK9 inhibitor represents a novel and practical approach to treat this target population.
CVI has developed CVI-LM001, an oral small molecule PCSK9 inhibitor, that is taken once a day and has sustained PK exposure. The company is also developing a first-in-class small molecule with novel metabolic targets from its library of over 300 novel compounds and plans to initiate human clinical studies in 2021.
About CVI Pharmaceuticals
CVI Pharmaceuticals is a clinical-stage biopharmaceutical company focused on building an innovative pipeline of first-in-class drugs to treat hypercholesterolemia and complex medical challenges of patients who suffer from liver and rare metabolic diseases. www.cvipharma.com.
www.cvipharma.com.