Product Pipeline
Overview
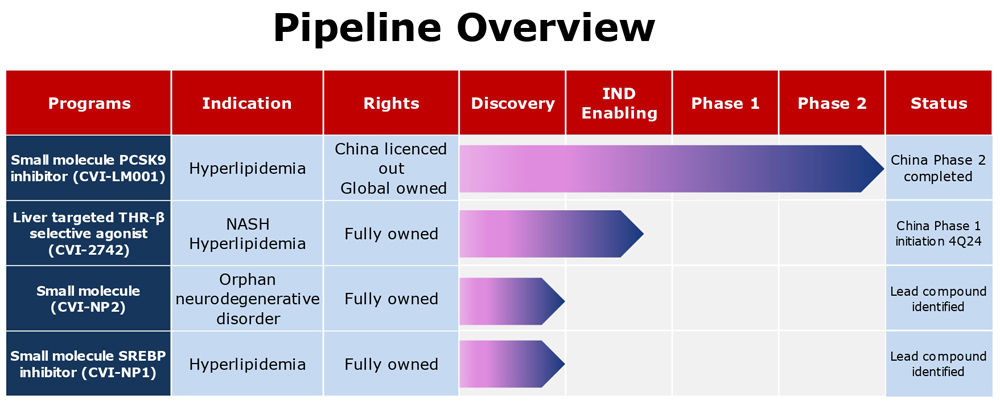
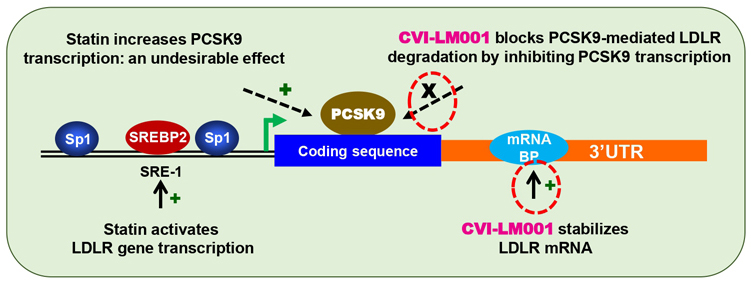
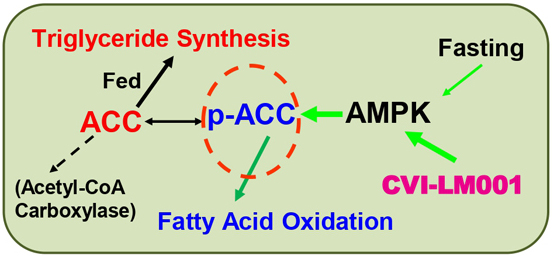
CVI-LM001 is a novel, first-in-class, safe and oral once daily PCSK9 modulator with distinct mechanisms of action in lowering blood atherogenic LDL-cholesterol and reducing liver fat respectively. Working synergistically with statins, CVI-LM001 has the potential to target patients with hypercholesterolemia and NAFLD/NASH patients with elevated LDL-C. Recently, a 12-week Phase 2 POC trial has successfully completed in China in patients with hypercholesterolemia.

Ask About Our Biopharmaceutical Product Candidates
If you wish to learn more about the research we’re conducting about new lipid-lowering mechanisms or have any questions about the pharmaceutical products we are developing, get in touch with us. Our team is more than happy to address your inquiries. You may contact us via the phone number or email address featured on this website. We hope to hear from you!